Ortho and para-nitrophenols are more acidic than phenol. Draw the resonance structures of the
corresponding phenoxide ions.
Or
Explain why p-nitrophenol is more acidic than phenol?
Resonating structures of o-nitrophenoxide ions that are formed by the loss of proton from o-nitrophenol are as follows:
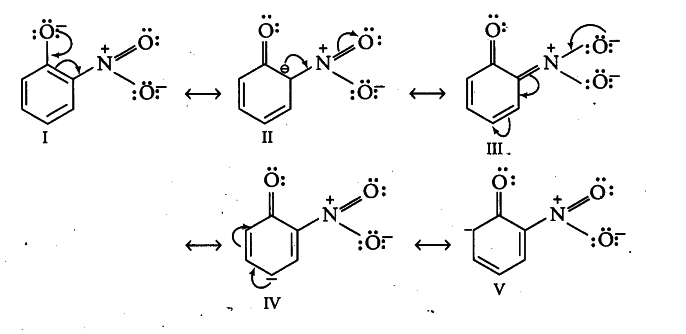
Resonating structures of p-nitrophenoxide ions that are formed by the loss of proton from p-nitrophenol are as follows:
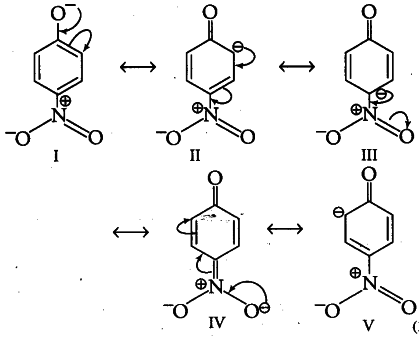
Resonating structures of phenoxide ions that are formed by the loss of proton from phenol are as follows:
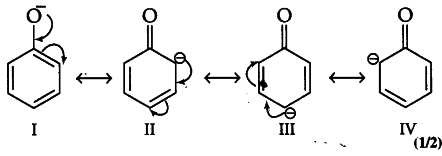
It is clearly evident from the above structures that due to —R-effect of— NO _{ 2 } group, o-and p-nitrophenoxide ions are more stable than phenoxide ions. Consequently, o- and p-nnitrophenols are more acidic than phenols.