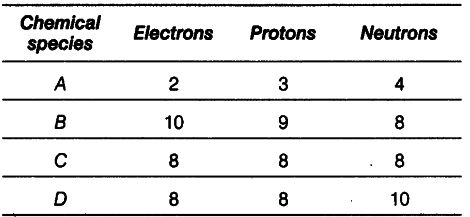
Number of electrons, protons and neutrors in chemical species A, B, C and D is given below.
Now answer the following questions.
(i) What is the mass number of A and B?
(ii) What is the atomic number of B?
(iii) Which two chemical species represent a pair of isotopes and why?
(iv) What is the valency of element C?Also justify your answers.
(i)Mass number of A = 3 + 4 = 7
Mass number of B = 9 + 8 = 17
(ii) Atomic number of B = Number of protons = 9
(iii) C and D are isotopes as they have same atomic
numbers but different mass numbers.
(iv) Electronic configuration of C : 2, 6(K,L)
It needs two electrons to complete its octet. Hence, its valency is 2.