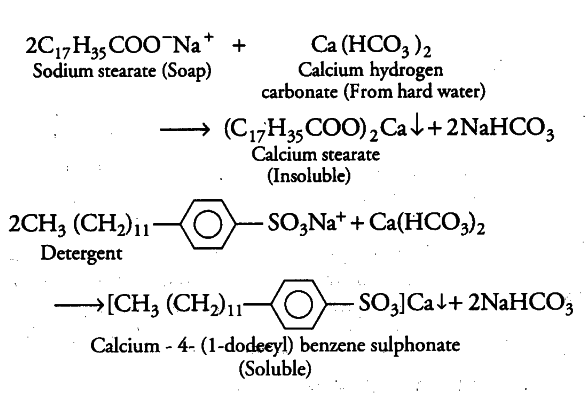
Water containing calcium hydrogen carbonate is hard water. Detergents are preferred over soaps for cleansing clothes in hard water. This is because in case of soaps calcium ions combines with soap to form
insoluble calcium salts of soap that separate as scum, and stick to the clothes as gummy mass while the calcium salts are soluble in water in case of detergents.