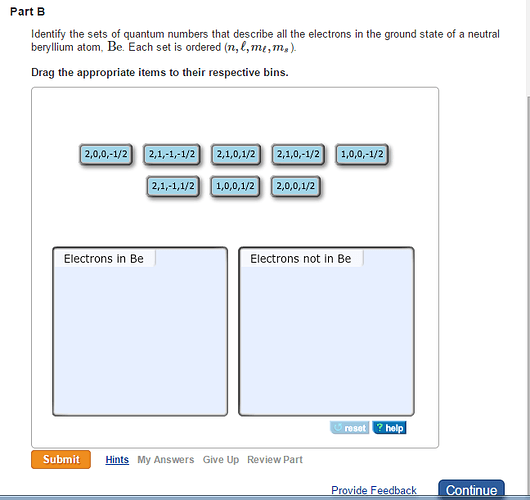
Identify which sets of quantum numbers are valid for an electron. Each set is ordered (n, l, ml, ms).
Concepts and reason
Four quantum numbers: (n, l,ml,ms)
Four quantum numbers are used to describe the most probable position of the electron of an atom in space.
The Pauli Exclusion Principle:
No two electrons can have the same set of four quantum numbers.
Hund’s Rule:
Electrons fill each orbital first in a subshell with one electron before entering half-filled orbitals.
Aufbau Principle:
Electrons will occupy the lowest energy level orbital first. This is used to determine the electronic configuration of an atom or ion.
Fundamentals
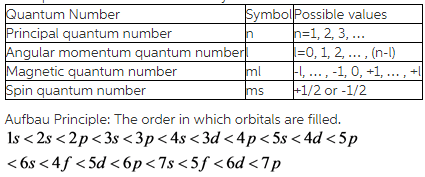
Four quantum numbers and their symbols:
Answer:
(A)


Explanation:
The valid sets of quantum numbers are identified and reasons are mentioned for invalid sets.
(B)
Symbolic representation of beryllium element with atomic number and mass number:
Beryllium = ![]()
It has totally four electrons.
Ground state electronic configuration of beryllium = ![]()

Explanation:
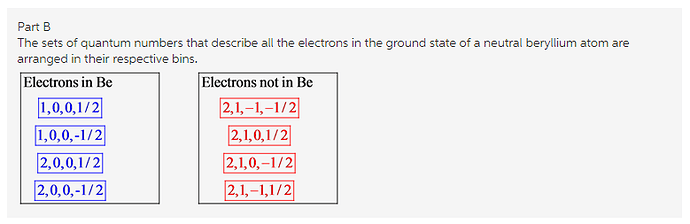
A neutral beryllium is found in a Group 2 (alkaline earth metals) family. It has two electrons in its core shell and two more electrons in its valence shell. The electronic configuration of a neutral beryllium atom is written as ![]() . The s electrons differ only in spin quantum numbers because the s subshell has one orbital. The principal quantum of 1s orbital is n=1 and 2s orbital is n=2. Thus, the sets of quantum numbers for four electrons in the ground state of a neutral beryllium atom are (1,0,0,1/2),( 1,0,0,-1/2),( 2,0,0,1/2) and (2,0,0,-1/2).
. The s electrons differ only in spin quantum numbers because the s subshell has one orbital. The principal quantum of 1s orbital is n=1 and 2s orbital is n=2. Thus, the sets of quantum numbers for four electrons in the ground state of a neutral beryllium atom are (1,0,0,1/2),( 1,0,0,-1/2),( 2,0,0,1/2) and (2,0,0,-1/2).