The electronic configuration of Al =
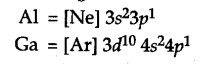
Gallium has 10 additional d-electrons in its inner shell or core of its atom. The presence of additional 10d electrons offer only poor screening effect for the outer electrons from the increased charge in gallium (Ga). Consequently, the atomic radius of gallium (135 pm) is less than that of aluminium (143 pm).