Give reason for the higher boiling point of ethanol in comparison to methoxy methane.
Or
Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Ethanol exists as associated molecules because ethanol undergoes intermolecular H-bonding due to the presence of hydrogen attached to the electronegative oxygen atom.
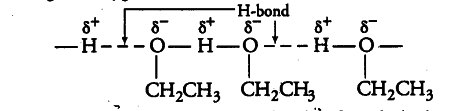
Thus, a larger amount of energy is required to break these H-bonds. Therefore, the boiling points of ethanol is higher than that of methoxy methane which does not form H-bonds.