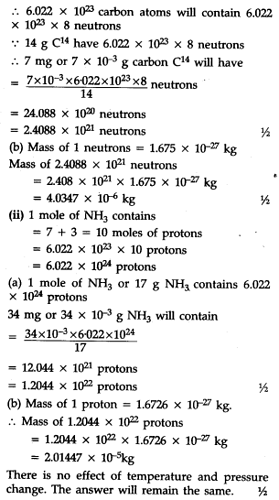
(i) Find (a) the total number and (b) die total mass of neutrons in 7 mg of ${}^{14}C$. (Assume that mass of a neutron = 1.675 x ${{10}^{-27}}$ kg)
(ii) Find (a) the total number and (b) the total mass of protons in 34 mg of N${{H}_{3}}$ at STP. (Mass of 1 p = 1.6726 X ${{10}^{-27}}$ kg) will the answer change if the temperature and pressure are changed ?