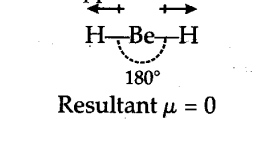
Due to sp hybridization shown by Be in Be${{H}{2}} the molecule Be{{H}{2}} is linear. Though the individual Be-H bonds are polar (due to difference of electronegativity) yet Be{{H}_{2}}$ has a dipole moment of zero as dipole moment is a vector quantity (it has both magnitude and direction). The individual polar character of Be-H bond cancels out as the dipoles are equal and opposite as shown below :