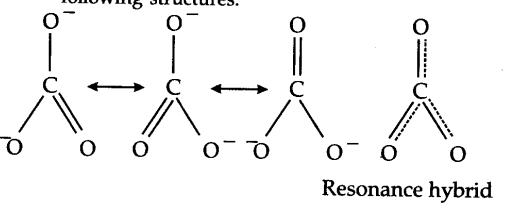
When a molecule cannot be represented by a single structure but its characteristic properties can be described by two or more than two structures, then the actual structure is said to be a resonance hybrid of these structures. For example, the carbonate ion may be represented as :
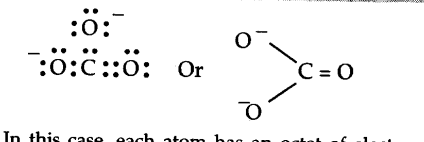
In this case, each atom has an octet of electrons. According to this structure, there are single bonds between two carbon—oxygen atoms and one double bond between carbon and oxygen atoms. Therefore, the two C—O bonds should be different than the third C = O bond. However, experimentally, it is observed that all the three bond lengths are equal and the bonds are intermediate between single and double bonds. This means that the above Lewis structure does not account for the observed experimental facts. To solve the problem, the C${{O}_{3}}^{2-}$ may be represented as a resonance hybrid of the following structures.