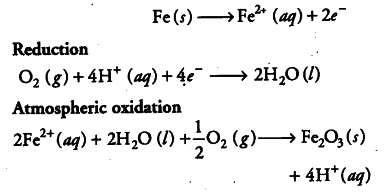In corrosion, a metal is oxidized by the loss of electrons to oxygen with the formation of oxides. So, an electrochemical cell is set-up, e.g. rusting of iron involves the following steps :
(i) The water layer present on the surface of iron dissolves acidic oxides from air like CO _{ 2 } and forms acid to produce {{H}^{+}} ions.
![]()
(ii) In the presence of {{H}^{+}} ions, iron starts losing electrons at some spot to form ferrous ions. The spot behaves as anode.
![]()
(iii) The electrons released at anode move to another spot where H+ ions and the dissolved oxygen gain these electrons. This spot becomes a cathode.
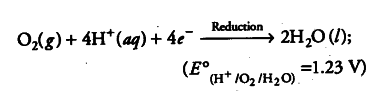
(iv) Overall reaction, i.e. redox reaction is
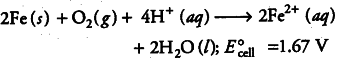
(v) Ferrous ions are further oxidised by the atmospheric oxygen to ferric ions which combine with water molecules to form hydrated ferric oxide (rust).
Oxidation