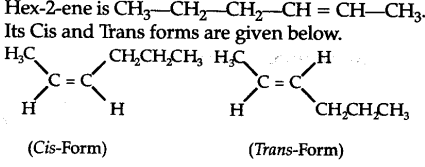
The cis isomer will have higher boiling point due to more polar nature leading to stronger intermo- lecular dipole-dipole interactions thus requiring more heat energy to separate them whereas trans from being non-polar (or weakly polar) have weak induced dipole interactions and so have lower boil-ing point.