Determine whether each of the following changes represents oxidation or reduction my answer: oxidation Reducatin NO2- to NH3 gain of electrons FADH2 to FAD ,NAD+ to NADH, CH4 to CO2 ,Ag+ to Ag, CH3CHO to CH3CH2OH, CH3OH to CH4, N2H4 to N2.
Concepts and reason
Chemical reaction that involves oxidation and reduction process can be explained in
- In terms of oxygen
- In terms of hydrogen
- In terms of electrons
Fundamentals
Oxidation and reduction
-
In terms of oxygen
The gain of oxygen is called oxidation.
The loss of oxygen is called reduction. -
In terms of hydrogen
The loss of hydrogen is called oxidation.
The gain of hydrogen is called reduction. -
In terms of electrons
The loss of electrons is called oxidation (increase in oxidation state).
The gain of electrons is called reduction (decrease in oxidation state).
Answer:
1.![]()
There is a loss of oxygen atoms as well as gain of hydrogen atoms therefore, this reaction is a reduction reaction.
2.![]()
There is a loss of hydrogen atoms therefore, this reaction is an oxidation reaction.
3.![]()
There is a gain of hydrogen atoms therefore, this reaction is a reduction reaction.
4.![]()
There is a gain of oxygen atoms as well as a loss of hydrogen atoms therefore, this reaction is an oxidation reaction.
5.![]()
There is a decrease in the oxidation number (that is, a gain of electrons) therefore, this reaction is a reduction reaction.
6.![]()
There is a gain of hydrogen atoms therefore, this reaction is a reduction reaction.
7.![]()
There is a loss of oxygen atom therefore, this reaction is a reduction reaction.
8.![]()
There is a loss of hydrogen atoms therefore, this reaction is an oxidation reaction.
Explanation:
Oxidation and reduction processes have been identified for the given reactions by observing the changes from reactants to products during the reaction.
Oxidation-reduction concept:
The gain of oxygen or loss of hydrogen or loss of electrons in a molecule during a reaction is called oxidation. Conversely, the loss of oxygen or gain of hydrogen atoms or gain of electrons during a reaction is called a reduction.
Oxidation and reduction processes for the given species are tabulated below.
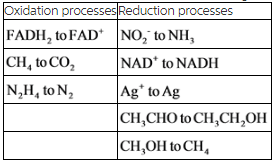
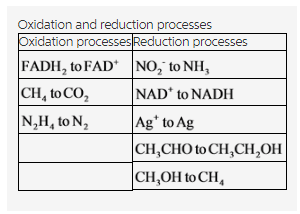
Explanation:
The oxidation and reduction processes have been classified for the given reactions. There are three oxidation and five reduction reactions in the given reactions.