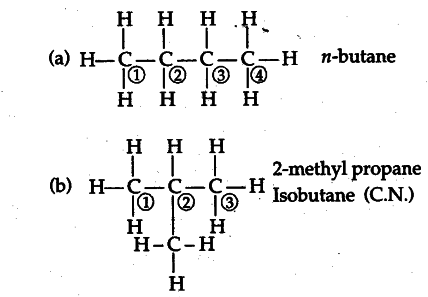
Define the term ‘structural isomerism’. Explain why propane cannot exhibit this property. Draw the structures of possible isomers of butane.
(i) The phenomenon due to which two or more such compounds exist which have same molecular formula but different structures.
(ii)Propane cannot exhibit this property, because due to lesser number of carbon atoms, it can form only one combination of atoms.
Isomers of butane :