Complete the following nuclear reactions
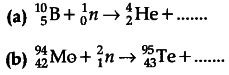
OR
If both the number of protons and neutrons in a nuclear reaction is conserved, in what way is mass converted into energy (or vice verse) ? Explain giving one example.
![]()
![]()
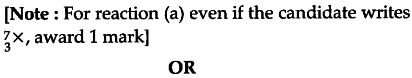
Since proton number and neutron number are conserved, the total rest mass of neutron and protons is the same on either side of the nuclear reaction. But total binding energy of nuclei on the left side need not be the same as that on the right hand side. The difference in binding energy causes a release of energy in the reaction. Example
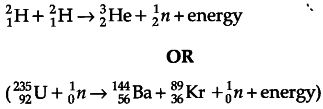
(Give full credit for any other one correct example.)