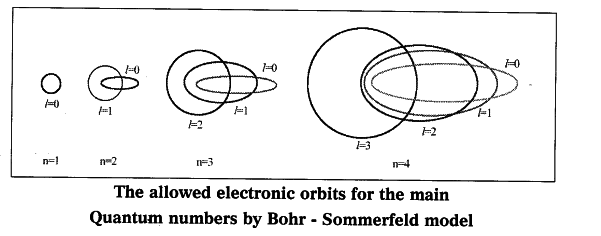
- In an attempt to account for the structure of line spectra, Sommerfeld modified Bohr’s atomic model by adding elliptical orbits.
- While retaining the first of Bohr’s circular orbit as such, he added one elliptical orbit to Bohr’s second orbit, two elliptical orbits to Bohr’s third orbit etc.
- Nucleus of the atom is one of the principal foci of these elliptical orbits because periodic motion under the influence of a central force will lead to elliptical orbits with the force situated at one of the foci.
Merit: Bohr - Sommerfeld model successful in accounting for the fine line structure of hydrogen atomic spectra.
Limitations : - This model failed to account for the atomic spectra of atoms of more than one electron.
- It did not explain Zeeman and stark effects.
Bohr’s Model of an Atom :
Niels Bohr proposed that electrons in an atom occupy ‘stationary’ orbits (states) of fixed energy at different distances from the nucleus.
When an electron ’jumps’ from a lower energy state (ground state) to higher energy states (excited state) it absorbs energy or emits energy when such a jump occurs from a higher energy state to a lower energy state.
The energies of an electron in an atom can have only certain values E _{ 1 }, E _{ 2 },
E _{ 3 }…; that is, the energy is quantized. The states corresponding to these energies
are called stationary states and the possible values of the energy are called energy levels.