Classify these compounds as acid, base, salt, or other. NaOH, KCl, NH3, HNO3, HCOOH, CO2, NaBr, and CH3CH3?
Concepts and reason
The problem is based on the concept of acid, base and salt.
Acids are the electron acceptor or proton donor while bases are the compounds which can donate electron pair or can accept proton. Salts are the ionic compounds which are formed by the neutralization reaction of acid and base.
Fundamentals
Acids are the chemical species which can readily accept electrons or can donate proton. In aqueous solution, acid donates the proton. Strong acids dissociates completely in the aqueous solution.
Bases are the chemical species which can readily donates an electron pair or hydroxyl ion or can accept proton. In aqueous solution, the base donates the hydroxyl ion. Salt are the neutral chemical compounds which are formed by the reaction between acid and base.
Answer:
Consider the chemical reaction of HCOOH in the aqueous solution as follows:
![]()
Consider the chemical reaction of ![]() in the aqueous solution as follows:
in the aqueous solution as follows:
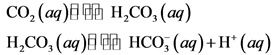
Consider the chemical reaction of ![]() in the aqueous solution as follows:
in the aqueous solution as follows:
![]()
![]()
Methanoic acid ![]() dissociates to give methanoate ion
dissociates to give methanoate ion![]() and protonin
and protonin ![]() the aqueous solution. The compound which gives proton are termed as acid. Therefore, is the acidic compound.
the aqueous solution. The compound which gives proton are termed as acid. Therefore, is the acidic compound.
Nitric acid ![]() dissociates to give nitrate ion
dissociates to give nitrate ion ![]() and proton
and proton ![]() in the aqueous solution. The compound which gives proton are termed as acid. Therefore,
in the aqueous solution. The compound which gives proton are termed as acid. Therefore,![]() is the acidic compound.
is the acidic compound.
In the aqueous solution, carbon dioxide![]() gives carbonic acid
gives carbonic acid ![]() which further dissociates to give hydrogen carbonate
which further dissociates to give hydrogen carbonate ![]() and proton
and proton ![]() . The compound which gives proton are termed as acid. Therefore,
. The compound which gives proton are termed as acid. Therefore, ![]() is the acidic.
is the acidic.
Consider the chemical reaction of NaoH in the aqueous solution as follows:
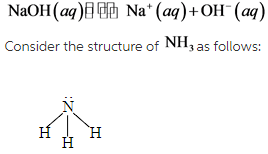
The molecule of ammonia has lone pair. Therefore, ![]() is a base.
is a base.
From the compounds,NaOH and ![]() are basic in nature.
are basic in nature.
