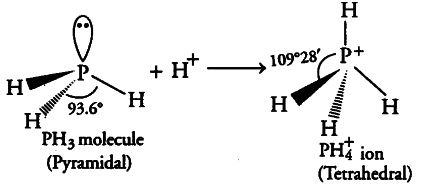
P in P{ H }_{ 3 } is sp3 hybridised. It has three bond pairs and one lone pair around P. Due to stronger lone pair-bond pair repulsions than bond pair-bond pair repulsions, the tetrahedral ai|gle decreases from 109°28’ to 93.6°. As a result, PH3 is pyramidal.
However, when it reacts with a proton, it forms P{ H }_{ 4 } ion which has four bond pairs and no lone pair. Due to the absence of lone pair-bond pair repulsions and presence of four identical bond pair-bond pair interactions, PH 4 assumes tetrahedral geometry with a bond angle ofl09°28’. Hence, bond angle in PH4 is higher than PH3.