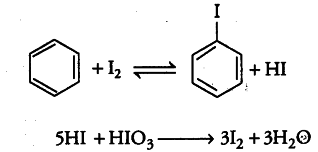
Aryl chlorides and bromides can easily be prepared by electrophilic substitution of arenes with chloride and bromide, respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires the presence of an oxidising agent?
Reactions of arenes with iodine are reversible in nature. So, aryl iodides are prepared in the presence of an oxidising agent such as HN{{O}_{3}}, HIO4, etc., in order to oxidise the HI formed during iodination.