Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
The hybridisation state of carbon in the given compounds is
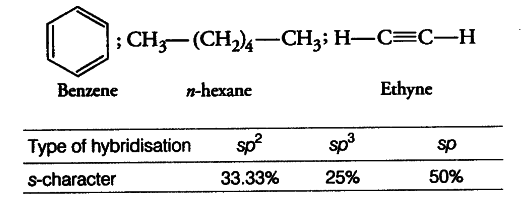
Acidic character increases with increase in s-character of the orbital. Hence, decreasing order of acidic behaviour of ethyne > benzene > hexane