(i) Among the second period elements, the actual ionization enthalpies are in the order.
![]()
Explain why (i) Be has higher A,H than B
(ii) O has lower A,H than N and F ?
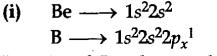
In case of Be electron has to be removed from an s-orbital whereas in Boron removal takes from p-orbital.
As s-orbital is closer to nucleus, hence removal of electron is difficult which raises its ionization enthalpy.
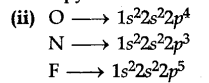
Comparing O and N, nitrogen has completely half filled p-orbitals, hence I.E. of nitrogen is greater than oxygen. [Half filled orbitals account for greater stability].
Comparing O and F, F has increased nuclear charge and small size, hence removal of electron is difficult. Therefore, I.E. F is greater than O.