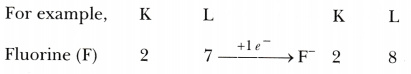Why group 17 elements form uninegative anions?
Answer:
Group 17 elements have 7 valence electrons, one electron less than the maximum number of electrons that can be accommodated in the outermost shell. Therefore, it is easier for these elements to gain an electron and form uninegative anions, so as to attain noble gas configuration.