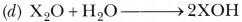The atomic number of an element ‘X’ is 19.
- Write its electronic configuration.
- To which period of the Modern Periodic Table does it belong and what is its valency?
- If ‘X’ burns in oxygen to form its oxide, what will be its nature - acidic, basic or neutral?
- Write balanced chemical equation for the reaction when this oxide is dissolved in water.
Answer:
- Configuration of X (19) = 2,8,8,1.
- It belongs to fourth period and its valency is 1.
- Basic oxide (X20)