State reason for the following:
- Lemon is used for restoring the shine of tarnished copper vessels.
- A metal sulphide is converted into its oxide to extract the metal from the sulphide ore.
- Copper wires are used in electrical connections.
Answer:
- When copper vessels are exposed to moist air, they form a green coating of basic copper carbonate [CuC03.Cu(0H)2].
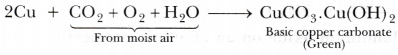
The sour substances such as lemon or tamarind juice contain acids. Lemon juice contains citric acid and tamarind contains tartaric acid. These acids dissolve the coating of copper oxide or basic copper carbonate present on the surface of tarnished copper vessels and make them shining red-brown again. - It is easier to obtain a metal from its oxides as compared to its sulphides and carbonates. So, prior to reduction, metal carbonate and sulphides must be converted into metal oxides. A carbonate ore is converted into oxide by calcination whereas a sulphide ore is converted into oxide by roasting.
- Copper wires are good conductor of electricity, so, they are used in electrical connections.