Give reasons:
(i) Conc. HN${{O}_{3}}$, can be transported in aluminium container.
(ii) A mixture of dilute NaOH and aluminium pieces is used to open drain.
(iii) Graphite is used as a lubricant
(iv) Diamond is used as an abrasive.
(v) Aluminium alloys are used to make aircraft
body.
Conc. HN${{O}{3}} can be transported in aluminium container because concentrated nitric add ( HN{{O}{3}}) renders aluminium passive by forming a protective 6xide layer on the surface.
<img src="/uploads/db3785/original/2X/7/7e7299bc5a6046c55f7656f7b7d33d201427b226.png" width="294" height="83">
A mixture of dU. NaOH and aluminium pieces is used to open drain becuase of the liberation of {{H}_{2}}$(gas)
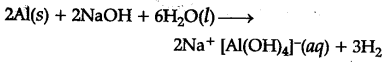
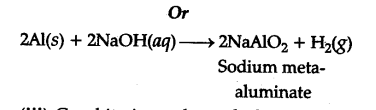
Graphite is used as a lurbicant because its
layers can slip over each other and therefore it is
very soft and slippery. It is used as a dry lubricant
in machines running at high temperature, where
oil cannot be used as a lubricant.
(iv) Diamond is used as an abrasive. The rigid
three-dimensional network of carbon atom extends
in space in diamond through strong covalent C-C
bonding. It is very difficult to break extended cova-
lent bonding and therefore, diamond is the substance on the earth. Therefore, it is used as an
abrasive for sharpening hard tools.
(v) Aluminum alloys are used to make aircraft body,
because its alloys with other metals makes it highly
tensile and due to its light weight nature it can be
given any shape like pipe, tube, roll, wire, plate or
foil.