Explain giving reason, which of the following sets of quantum numbers are not possible :
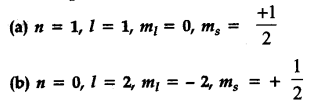
(a) when n = 1, value of l cannot be equal to 1.
Thus it is not possible.
(b) Value of n i.e., shell starts from 1 and not from zero. Thus, not possible.
Explain giving reason, which of the following sets of quantum numbers are not possible :
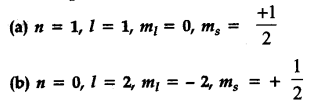
(a) when n = 1, value of l cannot be equal to 1.
Thus it is not possible.
(b) Value of n i.e., shell starts from 1 and not from zero. Thus, not possible.