B atom in B$C{{l}{3}}$ is sp2 hybridized due to which the shape of B$C{{l}{3}}$ is triangular planar. Anhydrous Al$C{{l}{3}} exists as dimer with the formula A{{l}{2}}$$C{{l}_{6}}$.
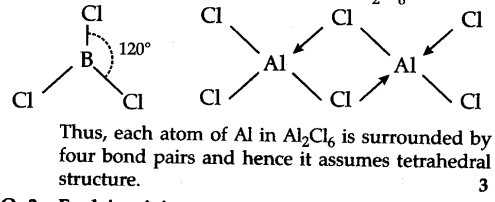
B atom in B$C{{l}{3}}$ is sp2 hybridized due to which the shape of B$C{{l}{3}}$ is triangular planar. Anhydrous Al$C{{l}{3}} exists as dimer with the formula A{{l}{2}}$$C{{l}_{6}}$.
