Atoms of seven elements A, B, C, D, E, F and G have a different number of electronic shells but have the same number of electrons in their outermost shells. The elements A and C combine with chlorine to form an acid and common salt respectively. The oxide of element A is liquid at room temperature and is a neutral substance, while the oxides of the remaining six elements are basic in nature. Based on the above information .
answer the following questions:
- What could the element A be?
- Will elements A to G belong to the same period or same group of the periodic table?
- Write the formula of the compound formed by the reaction of the element A with oxygen.
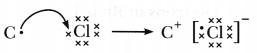
- Show the formation of the compound by a combination of element C with chlorine with the help of electronic structure.
- What would be the ratio of number of combining atoms in a compound formed by the combination of element A with carbon?
- Which one of the given elements is likely to have the smallest atomic radius?
Answer:
- Hydrogen
- Same group
- A2O/H2O
- 4:1
- A