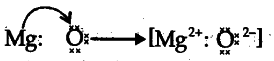An element is placed in 2nd Group and 3rd Period of the Periodic Table, bums in presence of oxygen to form a basic oxide.
(a) Identify the element.
(b) Write the electronic configuration
© Write the balanced equation when it bums in the presence of air.
(d) Write a balanced equation when this oxide is dissolved in water.
(e) Draw the electron dot structure for the formation of this oxide.
(a) Magnesium (Mg)
(b) K, L, M 2, 8, 2
© 2Mg(s) + O_{2}(g) ->2MgO(s)
(d) MgO(s) + $H_{2}$0(I) —> Mg(OH)_ {2}(aq)
(e)