When fossil fuels are burnt, a variety of pollutants | are emitted into the earth’s troposphere. Two of | the pollutants that are emitted are hydrocarbons (unburnt fuels) and nitric oxide (NO). When these j pollutants build up to suffidently high levels, a I chain reaction occurs from their interaction with sunlight in which NO is converted to nitrogen | dioxide (N${{O}{2}}). This N{{O}{2}} absorbs energy from sunlight and breaks up into nitric oxide and free oxygen atom.
<img src="/uploads/db3785/original/2X/d/db18f939655073724731bace09ca07a9b9caaf10.png" width="293" height="41">
Oxygen atoms are very reactive and can combine with the {{O}{2}} of the air to produce ozone.
<img src="/uploads/db3785/original/2X/9/9a845700cc2cf9d1722c0f34602c577a046195eb.png" width="251" height="34">
The ozone formed in the above reaction (H) reacts rapidly with the NO(g) formed in reaction (i) to regenerate N{{O}{2}}. N{{O}{2}}is a brown gas and at sufficiently high levels can contribute to haze.
<img src="/uploads/db3785/original/2X/b/b074e63541d5d5a27f451357c084e575be5f9ff4.png" width="343" height="36">
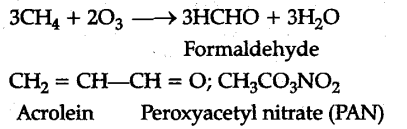
Ozone is a toxic gas and both N{{O}{2}} and {{O}{2}} are ) strong oxidising agents and can react with the unburnt hydrocabrons in the polluted air to produce chemicals such as formaldehyde (HCHO), acrolein C{{H}{2}}$ = CHCHO) and peroxyacety nitrate (PAN).