- Take dilute HCl in a beaker.
- Close it with a cardboard and introduce two different colour electrical wires through the holes made on it.
- Connect a bulb and make the connection as shown in the figure.
- Do the same replacing dilute HCl with dilute CH_{3}COOH (acetic acid). Observation :
The bulb glows brightly in HCl solution, while the bulb intensity is low in acetic acid solution.
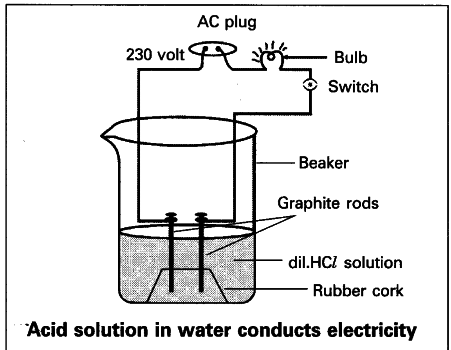
Result:
More ions are present in HCl solution which is a strong acid than in CH_{3}COOH solution which is a weak acid.