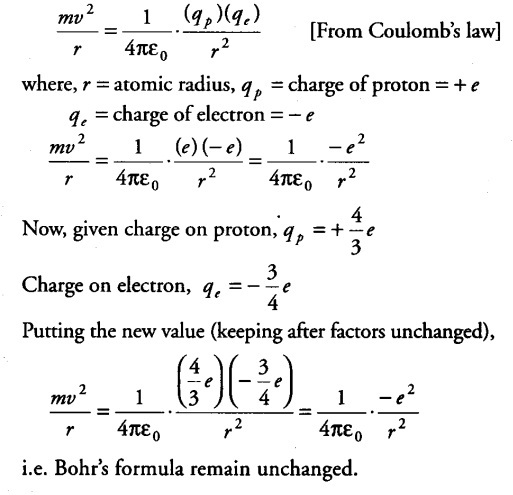
Would the Bohr’s formula for the H-atom remains unchanged, if proton had a charge (+ 4/3) e and electron had a charge (- 3/ 4)e, where e = 1.6 x ${{10}^{-19}}$ C . Give reasons for your answer.
According to Bohr’s theory, centripetal force required by the electron for its motion around the nucleus = Electric force between the proton and electron.