CC$C{{l}{4}}$ will not give a white ppt. of AgCl when it is treated with a solution of silver nitrate. It is because CC$C{{l}{4}} is a covalent compound and hence does not ionise to give CF ions which is the essential requirement for AgNC{{O}{3}} test.
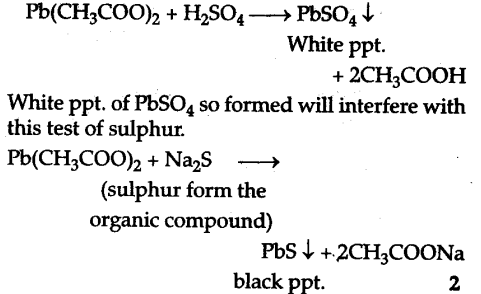
Why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in an organic compound ? Carbon dioxide (C{{O}{2}}) is slightly acidic in nature. Hence KOH which is a strong alkali dissolves C{{O}{3}} evolved from the compound containing carbon and reacts with it to form {{lK}{2}}C{{O}{3}}$. Increase in the weight of potash bulbs will give the weight of C$C{{O}{2}}$ evolved.