Why can we not determine the order of a reaction by taking into consideration the balanced chemical equation?
The rate of reaction may not depend upon all the molecules of a reactant present in the balanced chemical equation. Thus, balanced chemical equation often leads to incorrect order or rate law.
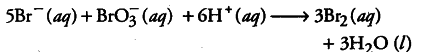
This is actually a fourth order reaction but it seems to be a twelfth order reaction. Actually, the reaction is complex and occurs in several steps. The order of such reaction is determined by the slowest step ih the reaction mechanism.