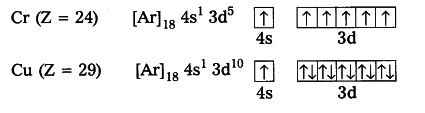
Elements which have half - filled or completely filled orbitals have greater stability.
So in chromium and copper the electrons in 4s and 3d redistributes their energies to attain stability by acquiring half-filled and completely filled d-orbitals.
Hence, the actual electronic configuration of chromium and copper are as follows.