Which pair of compounds will form a buffer in aqueous solution?
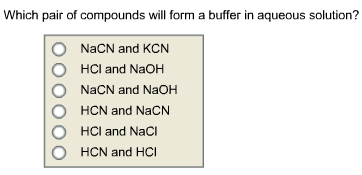
Answer:
A buffer solution is an aqueous solution of a weak acid and its conjugate base or weak base and its conjugate acid.
(a)
Since, both NaCN and KCN are salts and are neutral.
Therefore, NaCN and KCN cannot form a buffer solution.
Hence, this option is incorrect.
(b)
Since, HCI is a strong acid and NaOH is a strong base.
Therefore, HCI and NaOH cannot form a buffer solution.
Hence, this option is incorrect.



