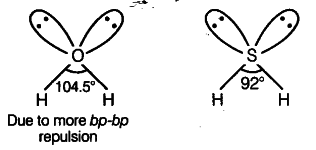
Out of $H _{ 2 }O$ and H2S, which one has higher bond angle and why?
Bond angle of H _{ 2 }O (H—O—H = 104.5°) is larger than that ofH2S (H—S — H = 92°) because oxygen is more electronegative than sulphur, therefore, bond pair electrons of O—H bond will be closer to oxygen which will be little a way from the sulphur atom.
As a result, bond pair-bond pair repulsions between bond pairs of two O—H bonds would be stronger than in the S—H bonds.