Which of the following reactions will be affected by increase in pressure ? Also mention whether this change will cause the reaction to go into forward or backward direction ?
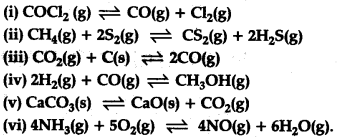
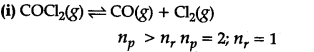
Equilibrium will shift to the backward direction on increasing pressure. No effect on increase of pressure.
(ii) In all the above reactions, the reaction no. (ii) proceeds with the same no. of moles on both the sides i.e ![]()
This reaction will not be affected by the increase in pressure, i.e., the direction of equilibrium will not be affected by the increase in pressure. All other reactions will be affected by the increase in pressure.
(iii)![]()
![]()
Equilibrium will go to backward direction on increase in pressure.
(iv)![]()
![]()
Equilibrium will shift to the forward direction on increasing pressure.
(v)
![]()
![]()
Equilibrium will shift backward direction on in¬creasing pressure.
(vi)![]()
![]()
Equilibrium shifts backward direction on increasing the pressure.