Which of the following reactions have a positive ?Srxn? Check all that apply.
2A(g) + B(s) --> 3C(g) 2A(g) + B(g) --> C(g) A(g) + B(g) -->C(g) 2A(g) + 2B(g) -->5C(g)
Concepts and reason
Entropy:
The entropy S describes the randomness and disorders of molecules, depending on the number of various arrangements possible for the molecules, in a given system of reaction.
Fundamentals
As the randomness of the molecules increases, the entropy also increases and gives a positive sign.
Conditions for ![]() to be positive:
to be positive:
- Entropy increases as the phase changes from solid to liquid, liquid to gas or solid to gas.
- If the change in number of moles is positive, then the entropy is also positive.
Answer:
The given reaction is as follows:
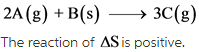
Explanation:
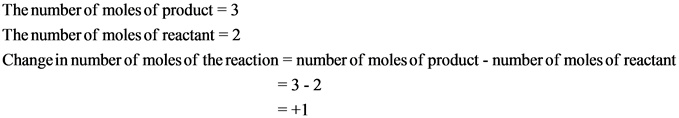
The change in number of moles is positive. Hence, the randomness of the molecules increases, which results in giving positive entropy.
The given reaction is as follows:
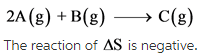
The change in number of moles is negative. Hence, the randomness of the molecules decreases, which results in giving negative entropy.
The given reaction is as follows:
![]()
Explanation:
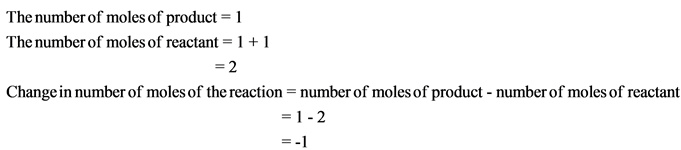
The change in number of moles is negative. Hence, the randomness of the molecules decreases, which results in giving negative entropy.
The given reaction is as follows:
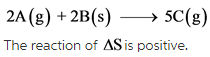
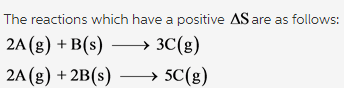
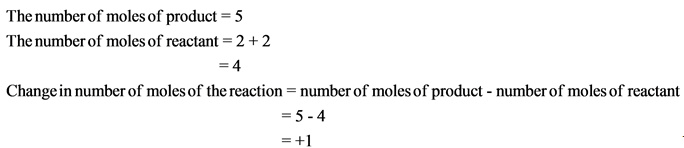
The change in number of moles is positive. Hence, the randomness of the molecules increases, which results in giving positive entropy.