Part A
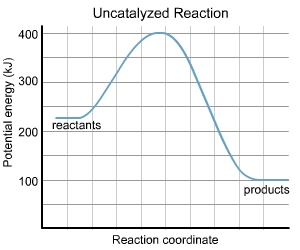
What is the value of the activation energy of the uncatalyzed reaction?
Express your answer to three significant figures and include the appropriate units.
Part B
What is the value of the enthalpy change of the uncatalyzed reaction?
Express your answer to three significant figures and include the appropriate units.
Part C
What is the value of the activation energy of the uncatalyzed reaction in reverse?
Part D
What is the value of the enthalpy change of the uncatalyzed reaction in reverse?
Express your answer to three significant figures and include the appropriate units.
Part E
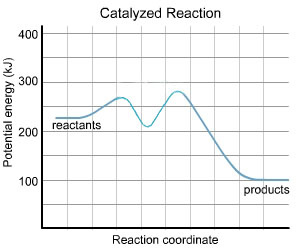
How does the presence of a catalyst affect the activation energy of a reaction?
How does the presence of a catalyst affect the activation energy of a reaction?

Part F
How does the presence of a catalyst affect the enthalpy change of a reaction?
How does the presence of a catalyst affect the enthalpy change of a reaction?

Answer:
For the uncatalysed reaction
The threshold energy = 400kJ
E reactants = 250kJ
E products = 100kJ
Thus activation energy Ea = Ethreshold - E reactans
and enthalpy of reaction Delta H = E products - E reactants
Part A
Activation energy for uncatalysed reaaction = E thre shold - E rreactant
= 400 -250 = 150kJ
Part B
enthalpy of reaction = 100-250
= - 150kJ
exothermic reaction
Part C for the reverse reaction
activation eenrgy = 400-100
= 300kJ
Part D
delta H for reverse reaction = 250 -100
= + 150 kJ
Part E
A catalyst lowers the activation energy of the reaction by altering the reaction pathway(mechanism)
Part F
The presence of catalyst does not change the enthalpy of reaction. It depnds on the energies of reactants andproducts which are unaffected by a catalst.