What is the threshold frequency ν0 of cesium? Note that 1 eV (electron volt)=1.60×10−19 J.
Express your answer numerically in hertz.
Concepts and reason
Calculate the threshold frequency by using the following equation.
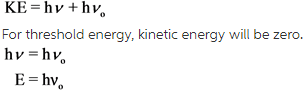
Here, h is plank constant,![]() is threshold frequency, and E is ionization energy.
is threshold frequency, and E is ionization energy.
Calculate the threshold frequency by using the values of plank constant and the energy.
Fundamentals
Minimum amount of frequency that causes photo electric emission is called as threshold frequency.
Emission of electrons when light is incident on surface of a material is called as photo emission and this effect is called as photo electric effect.
Answer:
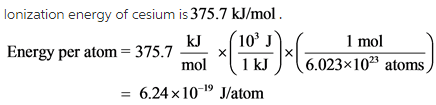
Minimum amount of energy required to remove an electron from an atom present in gaseous state is called as ionization energy.

Calculate the threshold frequency by substituting the values in ![]()
Substitute the values in the formula.

![]()
The value of plank constant in the units of kilograms is ![]() . Substitute
. Substitute ![]() for h and
for h and ![]() for E in the formula. Value of threshold frequency is obtained in the units of inverse of second. One inverse of second is equal to on hertz.
for E in the formula. Value of threshold frequency is obtained in the units of inverse of second. One inverse of second is equal to on hertz.
Threshold frequency of cesium atom is ![]() .
.