In a disproportionation reaction, same substance undergoes oxidation (increase in oxidation number) as well as reduction (decrease in oxidation number) resulting in the formation of two different products.e.g.
(i) Mn (VI) becomes unstable relative to Mn (VII) and Mn
(IV) in acidic solution.
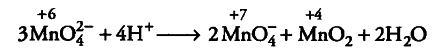
(ii) Many Cu (I) compounds are unstable in aqueous solution and undergo disproportionation as follows:
![]()