What happens when:
(a) Borax is heated strongly
(b) Boric acid is added to water
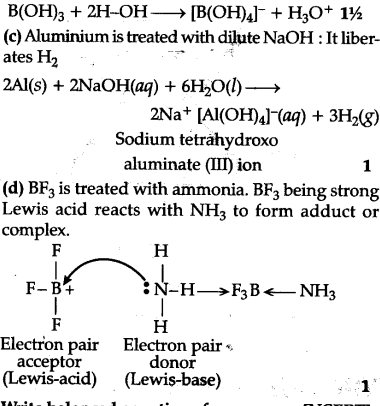
© Aluminium is treated with dilute NaOH
(d) BF3 is treated with ammonia ?
On heating borax it first loses water molecules and swells up. On further heating, it turns into a
transparent liquid which solidifies into glass like
material known as borax bead.
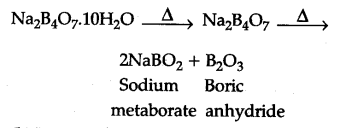
(b) Boric add is sparingly soluble in cold water but fairly soluble in hot water. Boric add behaves as a weak monobasic acid . It does not act as a proton-donor, i.e., protonic acid, but behaves as a Lewis-acid, i.e., it accepts a pair of electrons .