What are soaps chemically ? How do they differ from synthetic detergents ? Explain the mechanism and the cleansing action of soap.
(i) Soap : It is sodium or potassium salt of fatty acid.
Detergents are ammonium or sulphonate salts of long chain carboxylic acids.
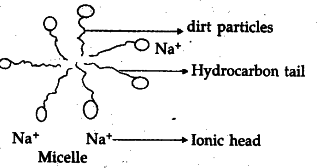
(ii) Due to micelle formation, ionic - ionic repulsion.
Micelle : It is a structure formed when soap molecules get arranged and align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water.