Two carbon compounds A and B have molecular formula $C _{ 3 }$$H _{ 8 }$ and $C _{ 3 }$$H _{ 6 }$
respectively. Which one of the two is most likely to show addition ? Justify your answer.
Out of $C _{ 3 }$$H _{ 8 }$ and $C _{ 3 }$$H _{ 6 }$ , the latter is an unsaturated hydrocarbon and thus it will give addition reaction. For example, C3H6 adds up bromine or hydrogen as follows.
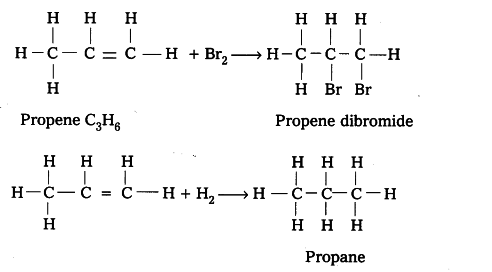
Oils are unsaturated hydrocarbons. When treated with hydrogen, addition reaction takes place and a saturated mass is obtained.
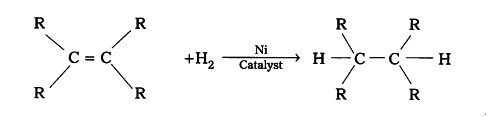
Where R is a long chain alkyl part.