Three vessels of equal capacity have gases at the same temperature and pressure. The first vessel contains neon (monatomic), the second contains chlorine (diatomic) and the third contains uranium hexafluoride (polyatomic). Do the vessels contain equal number of respective molecules? Is the root mean square speed of molecules the same in the three cases? If not, in which case is the largest?
As three vessels are identical i.e., they have same volume now at constant pressure, temperature and volume the three vessels will contain equal number of molecules (by Avogadro’s law) and is equal to Avogadros number,
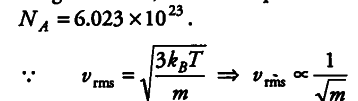
where m is mass of single gas molecule as neon has the smallest mass so rms speed will be greatest in case of neon