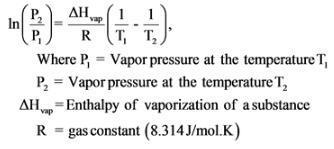
The vapor pressure of a liquid is thepressure exerted by its vapor when the liquid and vapor states are in equilibrium. The relationship between vapor pressure, P, and temperature, T, is expressed by the Clausius-Clapeyronequation
![]()
Part A.
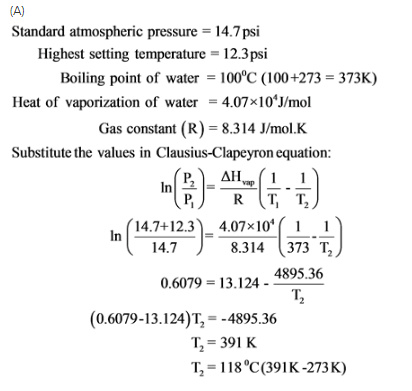
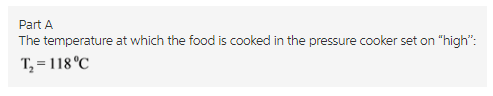
The instruction booklet for your pressure cooker indicates thatit’s highest setting is 12.3 psi. You know that standard atmosphericpressure is 14.7 \rm psi, so the booklet must mean12.3 psi above atmospheric pressure. Atwhat temperature will your food cook in this pressure cooker set on"high"?
Express your answer numerically indegrees Celsius.
![]()
Part B.
Which of the following factors affect thevapor pressure of a liquid?
Choose all that apply.
a) amount of liquid
b) atmospheric pressure
c) addition of solute
d) surface area of the liquid
e) type of liquid
f) temperature
Concepts and reason
Vapor pressure is the pressure exerted by the vapor in equilibrium with its condensed phase (liquid or solid) in a closed system at the given temperature.
Fundamentals
The relationship between temperature and pressure is expressed with the help of the Clausius – Clapeyron equation:
Answer:
(B)
a)Amount of liquid: The vapor pressure of a liquid does not change with the amount of liquid present. Therefore, there is no effect on vapor pressure.
b)Atmospheric pressure: The vapor pressure of a liquid does not depend on atmospheric pressure. Therefore, it has no effect on vapor pressure.
d)Surface area of the liquid: The vapor pressure of the liquid has no effect on the surface area of the liquid.
c)Addition of solute: The vapor pressure of the liquid changes on adding solute.
The vapor pressure changes on the type of liquid present.
Vapor pressure increases on increasing the temperature.
