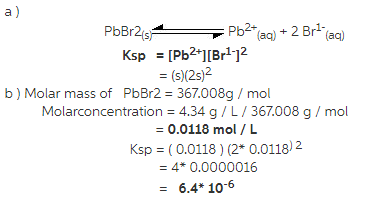The solubility of lead(II) bromide, PbBr2, in water is4.34 g/L. In an aqueous solution an equilibrium exists between thedissolved ions and the solid salt.
![]()
1) write the solubility product expression for thisequation.
2) calculate the molar concentration and theKsp ( solubility product constant) for lead (II)bromide.
Answer: