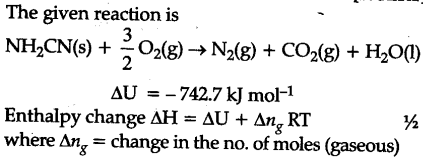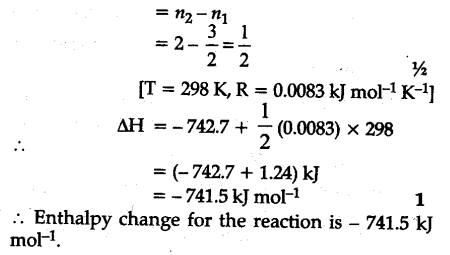The reaction of cyanamide N${{H}{2}}$CN(s) with ${{O}{2}}$ was carried out in a bomb calorimeter and AU was found to be - 742.7 kj/mol at 298 K. Calculate enthalpy change for the reaction at 298 K
N${{H}{2}}$CN+3/2${{O}{2}}$------>N${{O}{2}}$+C${{O}{2}}$+${{H}_{2}}$O