Table given below shows a part of the Periodic Table:
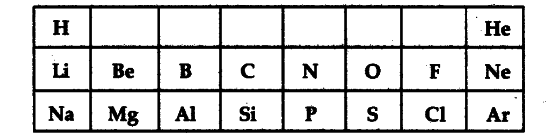
Using this table, explain why :
(a) Li and Na are considered as active metals.
(b) Atomic size of Mg is less than that of Na.
© Fluorine is more reactive than chlorine.
Table given below shows a part of the Periodic Table:

Using this table, explain why :
(a) Li and Na are considered as active metals.
(b) Atomic size of Mg is less than that of Na.
© Fluorine is more reactive than chlorine.
(a) Because they have only one valence electron which they can lose easily and thus show high reactivity.
(b) In a period nuclear charge increases which leads to increased attraction between nucleus and the electrons resulting in decrease in size.
© The reactivity of non-metals depends upon the tendency to gain electrons since fluorine is smaller in size and have more nuclear charge as compared to chlorine. Therefore, it accepts the electron more easily than chlorine and thus is more reactive.