Study the variation in the atomic radii of first group elements given below and arrange them in an increasing order.
(i) Name the elements which have the smallest and the largest atoms.
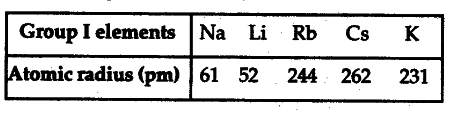
(ii) How does the atomic size vary as you go down a group?
Study the variation in the atomic radii of first group elements given below and arrange them in an increasing order.
(i) Name the elements which have the smallest and the largest atoms.

(ii) How does the atomic size vary as you go down a group?
(i) The element having the smallest atom is lithium (Atomic radius is 52 pm) while the element . having the largest atom is caesium (Atomic radius is 262 pm).
(ii) The atomic size increases while going down a group because new shells are being added at each step.